Chemistry Question #2087
Bob Salo, a 62 year old male from Michigan asks on May 10, 2004,
If matter expands when heated, and contracts when cooled, why does ice expand when water freezes? Please explain in layman terms.
viewed 23902 times
The answer
Barry Shell
answered on May 10, 2004
The basic idea is that water molecules are V shaped with about 105 degrees separating the two arms of the V. There's an oxygen at the bottom of the V and one hydrogen at the end of each arm of the V. When water freezes, it goes from a mixed up liquid state where all these V's are just sliding around each other, to an ordered crystalline solid state where all the V's have to connect with each other in nice orderly solid shapes.
Illustrations by Raymond Nakamura.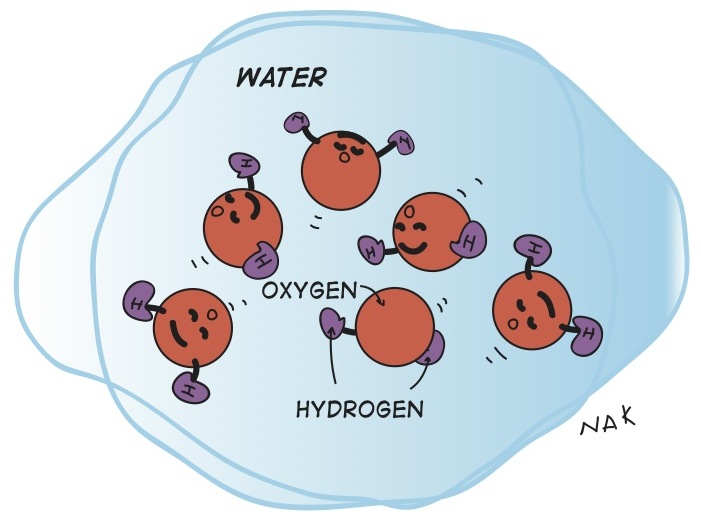
The closest and easiest solid crystal shape for something that exists as a 105 degree V is a hexagonal (really tetrahedral in 3D) crystal. Think of it as a flat hexagonal snowflake shape, but it really goes in three dimensions. To get this shape, the water molecule V angle has to open up a bit to about 108 degrees. (109.47 degrees would be the ideal angle, but it doesn't make it quite this far in real life.)
 The water molecules want to do this because to them it "feels" nicer--that is: they feel less strain and they can get into a lower energy state by getting into this nice orderly hexagonal crystal. Think of it as though it's more "relaxing" to the water molecules to open up the V angle a bit as they turn from water to solid ice. Anyway, because of this "opening up", the distance between molecules is a bit bigger. In fact, taking into consideration some other things about bond distances, the openness of ice is about 9% bigger than liquid water. In other words: in the same given volume of ice there are less water molecules than in liquid water, because each one is just a bit bigger in ice than when it is liquid. So that is why ice is lighter than liquid water.
The water molecules want to do this because to them it "feels" nicer--that is: they feel less strain and they can get into a lower energy state by getting into this nice orderly hexagonal crystal. Think of it as though it's more "relaxing" to the water molecules to open up the V angle a bit as they turn from water to solid ice. Anyway, because of this "opening up", the distance between molecules is a bit bigger. In fact, taking into consideration some other things about bond distances, the openness of ice is about 9% bigger than liquid water. In other words: in the same given volume of ice there are less water molecules than in liquid water, because each one is just a bit bigger in ice than when it is liquid. So that is why ice is lighter than liquid water.
By the way, of nearly 15 million substances we know about, water is one of a very few that expand when freezing. Because of this and a number of other phenomena mostly based on this V angle thing and in particular on something called "Hydrogen Bonding", water is probably the most amazing substance in the universe. I'm not exagerating. Other websites have some excellent pictures and explanations of water shape and behaviour.
Add to or comment on this answer using the form below.
Note: All submissions are moderated prior to posting.
If you found this answer useful, please consider making a small donation to science.ca.